Hybrid lead halide DMAPbX3 hexagonal perovskites
15 Jan 2020Article: Hybrid lead halide [(CH3)2NH2]PbX3 (X = Cl- and Br-) hexagonal perovskites with multiple functional properties
 |
|---|
| Hybrid lead halide DMAPbX3hexagonal perovskites with multiple functional properties |
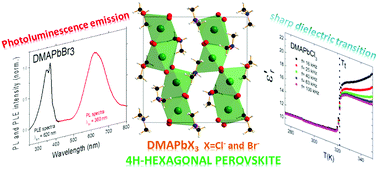
We have been able to prepare two new lead halides with the formula DMAPbX3 (where DMA is dimethylammonium and X is Cl- and Br-), which display a 4H-hexagonal perovskite polytype, an unusual crystal structure in hybrid organic–inorganic perovskites. Both compounds experience a first-order phase transition, the chloride at ∼320 K and the bromide at ∼250 K, which involves two cooperative processes: an off-center shift of the lead cations and an order-disorder process of the DMA cations. Additionally, a sharp dielectric transition is associated with this structural transition in both hybrids. Both compounds are semiconductors with band gap values of 3.5 eV (X: Cl- ) and 3.0 eV (X: Br-). Also, the LT-phase of the Br- compound displays a broad red light photoluminescence (PL) emission (620 nm) and PLE excitation with the maximum at a soft UV wavelength (360 nm). This broadband emission and large Stokes-shifted PL seem to be related to a self-trapped exciton mechanism. Therefore, the uncommon 4H-hexagonal perovskite is a promising crystal structure for understanding and designing new hybrid materials with broad photoluminescent emission and dielectric properties.
The pseudopotentials used with the Siesta program can be downloaded here.
