Thermal degradation of CH3NH3PbI3 perovskite into NH3 and CH3I gases
29 Dec 2016Article: Thermal degradation of CH3NH3PbI3 perovskite into NH3 and CH3I gases observed by coupled thermogravimetry mass spectrometry analysis
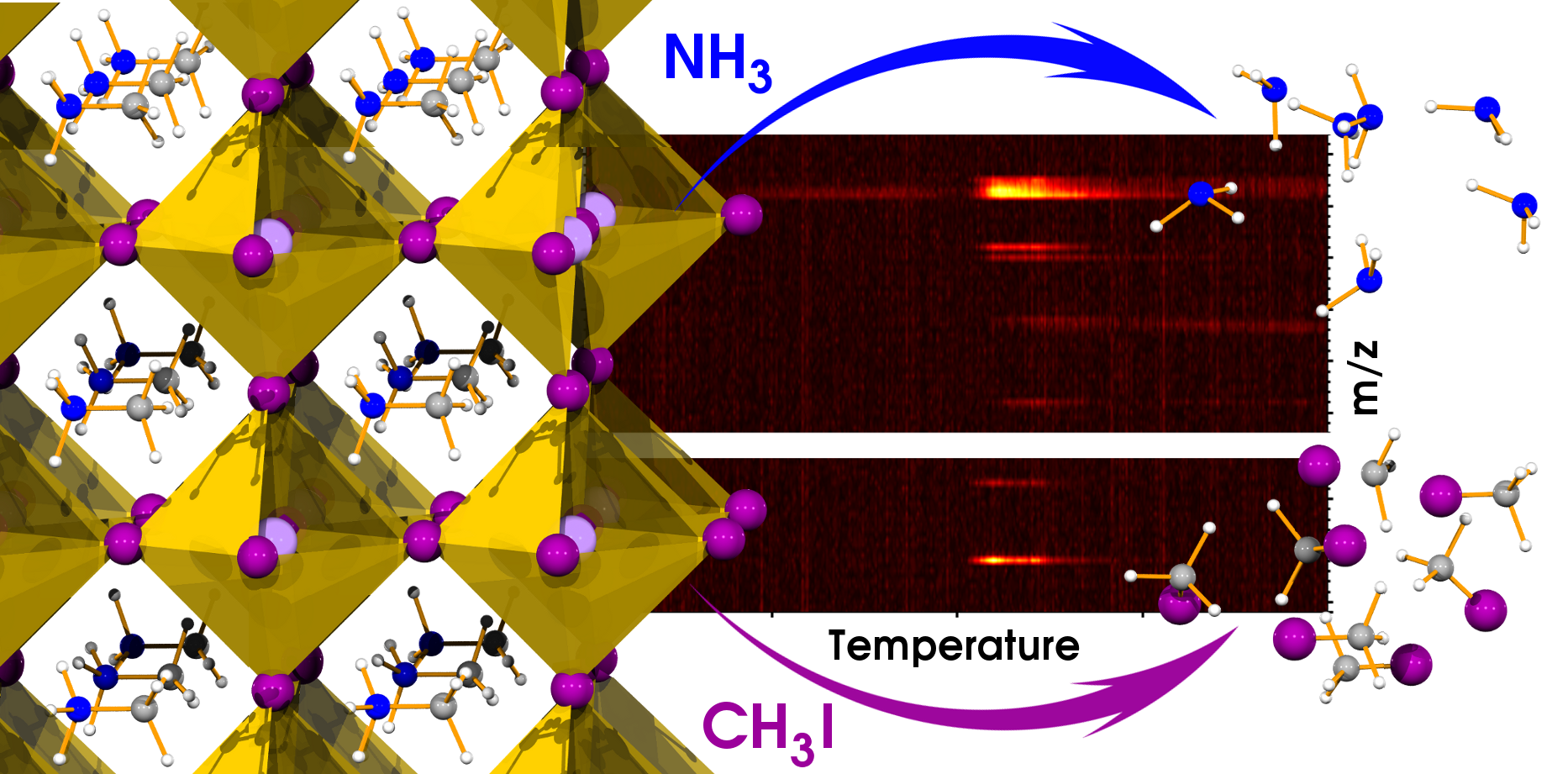
Thermal gravimetric and differential thermal analysis (TG-DTA) coupled with quadrupole mass spectrometry (MS) instrumentation and first principles calculations were employed to elucidate the chemical nature of released gases during the thermal decomposition of MAPbI3. Contrarily to the common wisdom that CH3NH3PbI3 is decomposed into CH3NH2 and HI, the major gases were methyliodide (CH3I) and ammonia (NH3). We anticipate our finding will provide new insights for further formulations of the perovskite active material and device design that can prevent the methylammonium decomposition and thus increase the long-term stability of perovsktie-based optoelectronic devices.
This article called the attention of the specialized media: Altmetrics
 |
|---|
| Graphical Abstract figure for the TOC |
 |
|---|
| Figure used in the Back cover of the issue. Program: Blender |
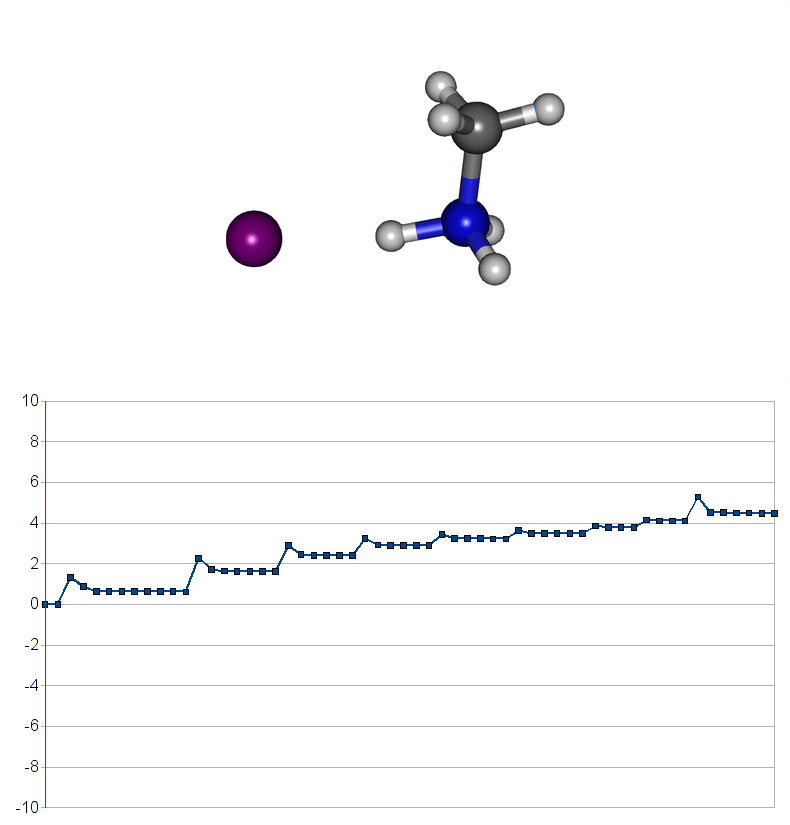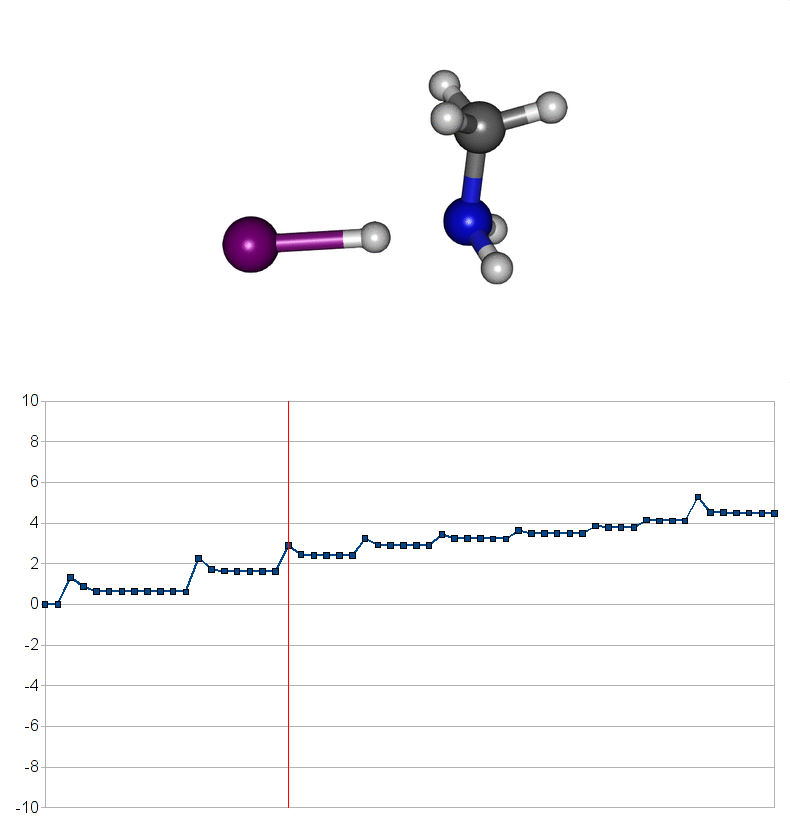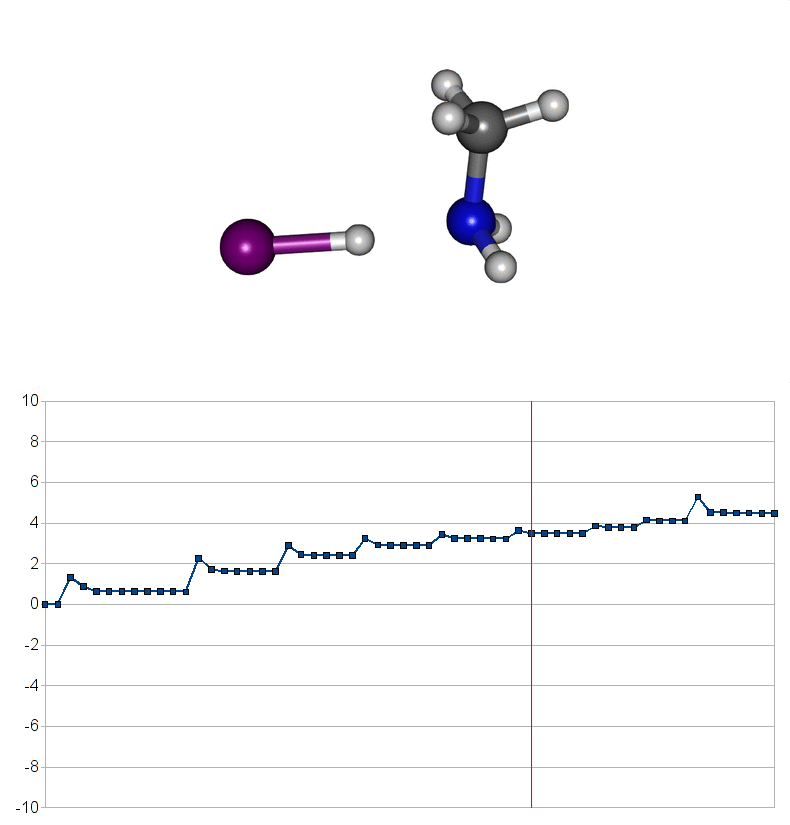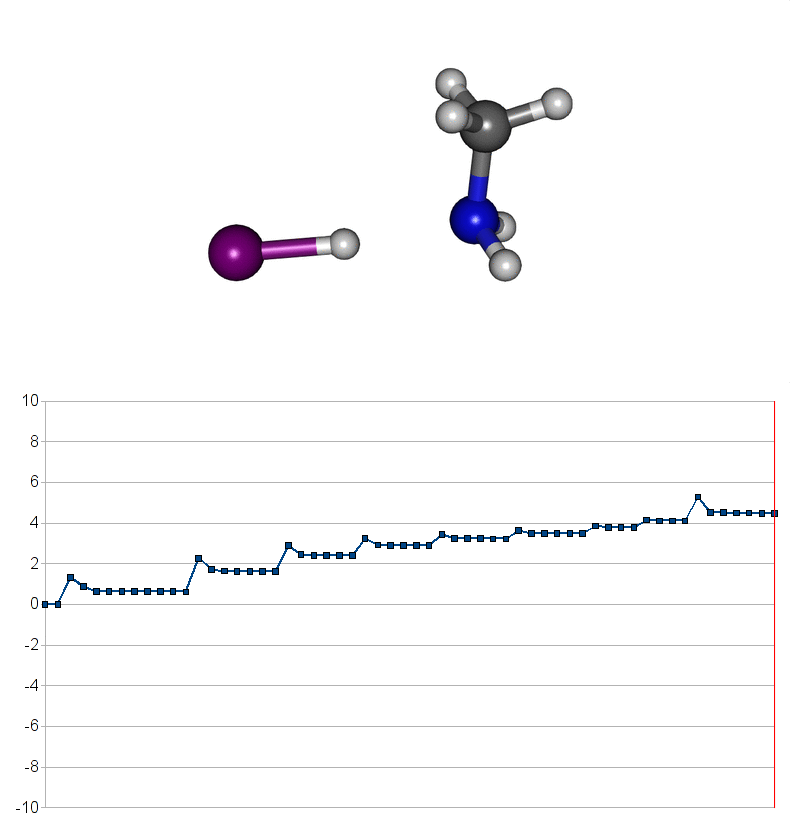
Two energy pathways for methylammonium iodide decomposition depending of released products
 |
|---|
| Energy consuming reaction for decompostion into CH3NH2 and HI |
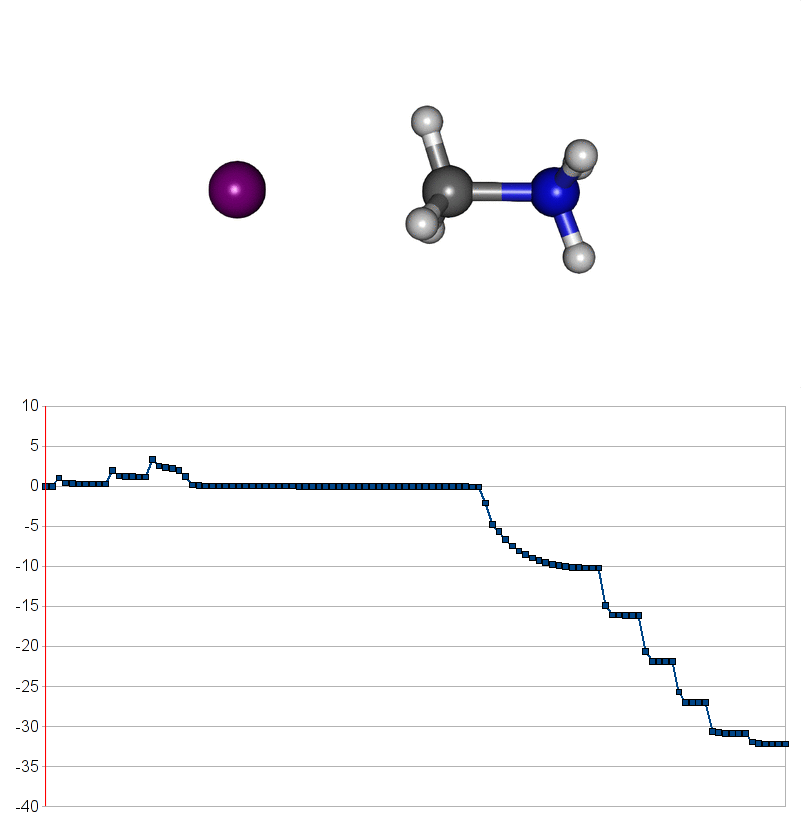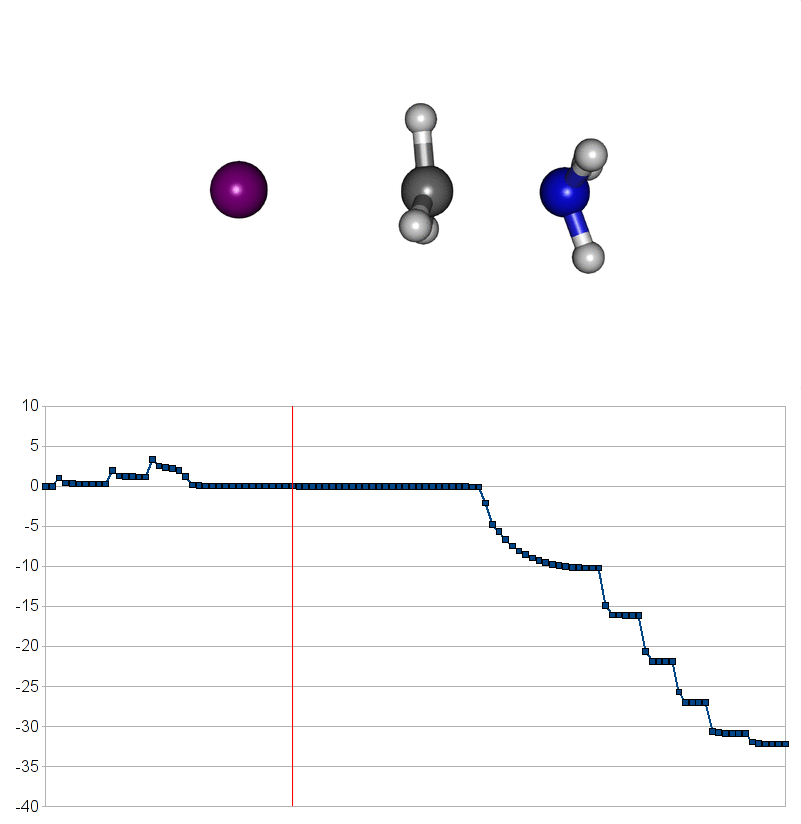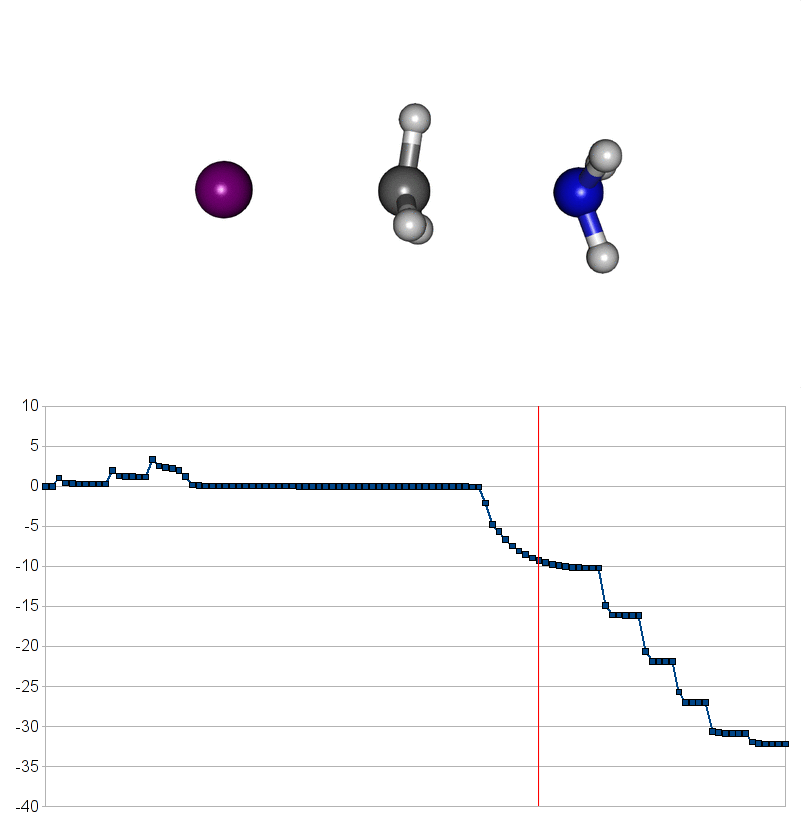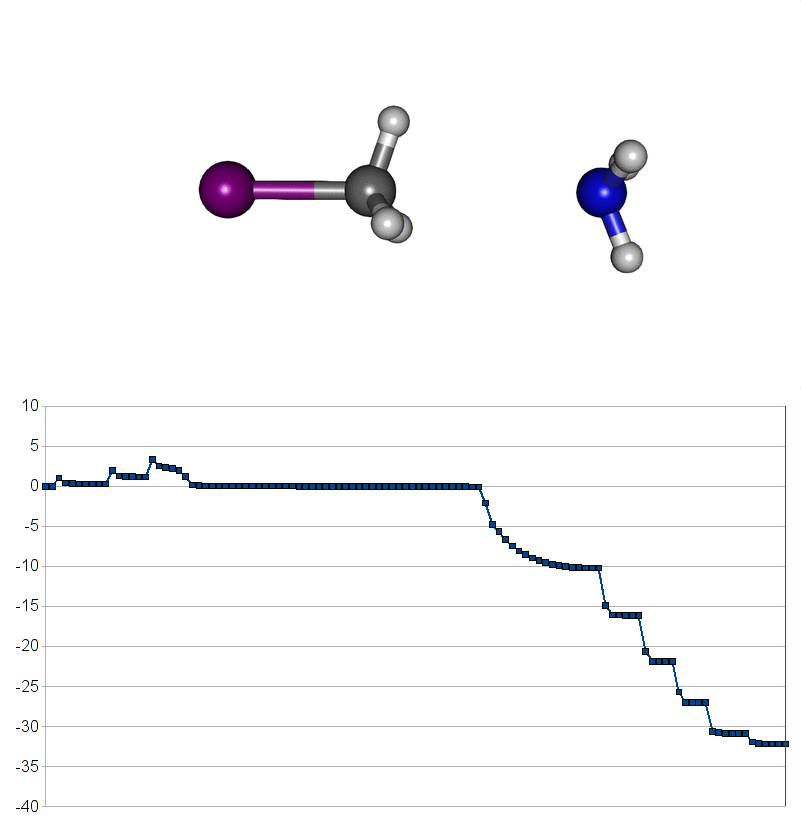 |
|---|
| Energy releasing reaction for decompostion into methyliodide (CH3I) and ammonia (NH3) |
