Photo-,thermal-decomposition in methyalammonium based perovskite
12 Jun 2018
Hybrid lead halide perovskites have emerged as promising active materials for photovoltaic cells. Although superb efficiencies have been achieved, it is widely recognized that long-term stability is a key challenge intimately determining the future development of perovskite-based photovoltaic technology. Herein, we present reversible and irreversible photodecomposition reactions of methylammonium lead iodide (MAPbI3). Simulated sunlight irradiation and temperature (40-80 ºC) corresponding to solar cell working conditions lead to three degradation pathways: (1) CH3NH2+ HI (identified as the reversible path), (2) NH3+ CH3I (the irreversible or detrimental path), and (3) a reversible Pb(0) + I2(g) photodecomposition reaction. If only the reversible reactions and take place and reaction can be avoided, encapsulated MAPbI3can be regenerated during the off-illumination timeframe. Therefore, to further improve operational stability in hybrid perovskite solar cells, detailed understanding of how to mitigate photodegradation and thermal degradation processes is necessary. First, encapsulation of the device is necessary not only to avoid contact of the perovskite with ambient air, but also to prevent leakage of volatile products released from the perovskite. Second, careful selection of the organic cations in the compositional formula of the perovskite is necessary to avoid irreversible reactions. Third, selective contacts must be as chemically inert as possible toward the volatile released products. Finally, hybrid halide perovskite materials are speculated to undergo a dynamic formation and decomposition process; this can gradually decrease the crystalline grain size of the perovskite with time; therefore, efforts to deposit highly crystalline perovskites with large crystal sizes may fail to increase the long-term stability of photovoltaic devices.
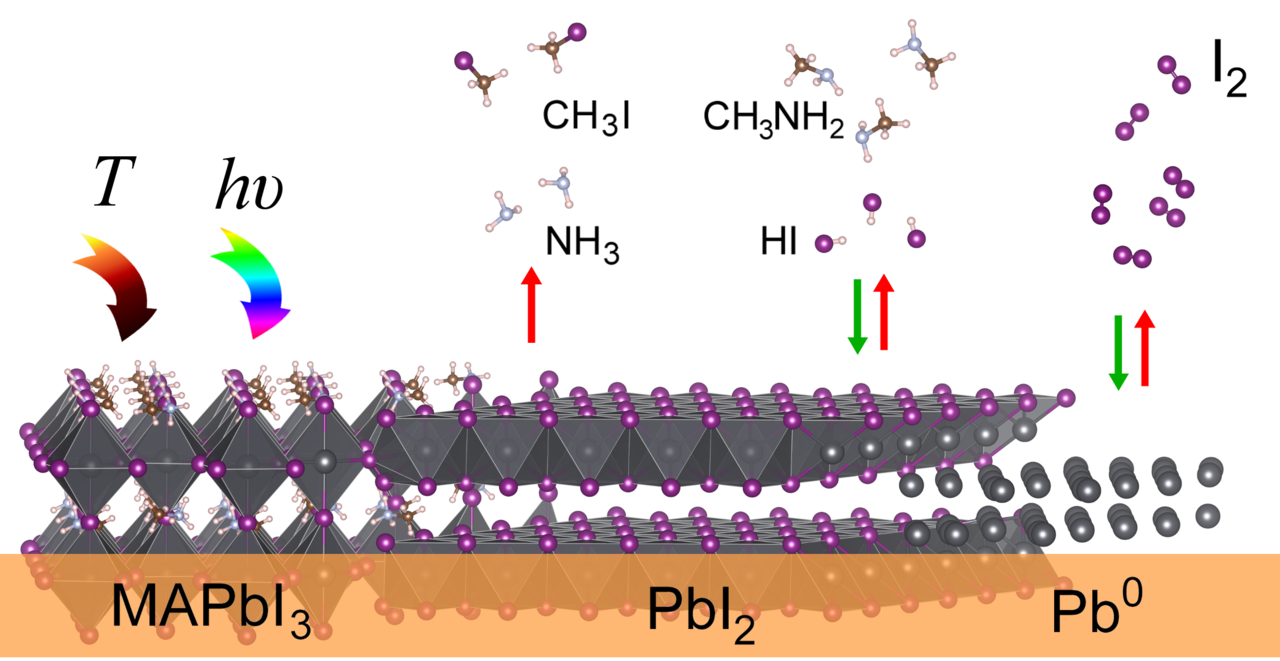 |
| Scheme depicting several routes (reversible and irreversible) for MAPbI3 decomposition under temperature and lightning factors |
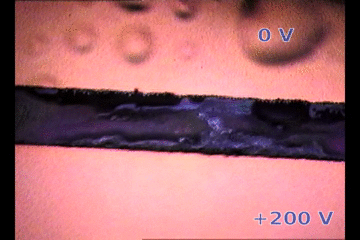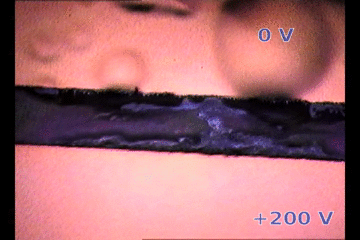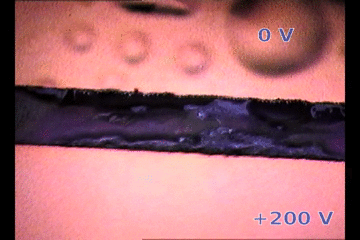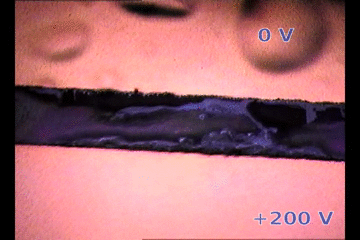 |
| Parylene/Gold cathode “bubbling” on top of a biased perovskite thin film releasing gases. The perovksite fringe between electrodes is ~100 micrometers width. 200V/100um is an electric field similar to suffered by 1 um thick perovskite layer on solar cells |


